 View full size
View full size
- Nucleic Acid Analysis
- Protein Analysis
- Biochemical Reagents
-
Enzymes
- Thermophilic DNA Polymerases
- Mesophilic DNA Polymerases
- Restriction Endonucleases
- Reverse Transcriptase and RNA Polymerases
- DNA/RNA Ligases
- RNases
- Proteases
- Nucleases
- Kinases
- Phosphatases and Sulfurylases
- DNA Repair Proteins
- Single-Stranded DNA Binding Proteins
- Chaperon Proteins and Disulfide Bond Isomerase
- Others
- Gene editing
- Molecular cloning
- Clinical diagnostics
- Human Identification STR kits
- Laboratory instruments
- Software
- A&A Biotechnology
- AdvancedSeq
- BioDynami
- Plant Cell Technology
News
-
RANK 2026
Visit us at the jubilee 20th edition of the RANK 2026 professional conference, which will take place on March 18–19 in Pardubice at the ABC Club. The conference is organized by the Czech Society of Cl...
Read more -
XXXV. Izakovičov memoriál 2025
We are pleased to announce our participation in the prestigious XXXV. Izakovič Memorial 2025, which will take place on October 8–10, 2025 at the Grandhotel Praha, Tatranská Lomnica. The Izakovič Memo...
Read more -
1st Czechoslovak Congress of Medical Genetics 2025
In the spring, we will participate in the 1st Czechoslovak Congress of Medical Genetics, which will take place from April 2–4, 2025, at the Cultural and Congress Center Elektra in the spa town of Luha...
Read more
 View full size
View full size
Description:
The AmoyDx® Pan Lung Cancer PCR Panel (PLC Panel) is a tissue-based, real-time, qPCR assay for in vitro diagnostics (IVD). The PLC Panel enables qualitative detection of up to 167 variants (85 DNA mutations and 82 RNA fusions) in 11 genes (EGFR, ALK, ROS1, KRAS, BRAF, HER2, RET, MET, NTRK1, NTRK2, and NTRK3), identifying all NCCN recommended biomarkers in a single PCR run. The PLC Panel is intended to assist in identifying clinically relevant biomarkers for patients with non-small cell lung cancer (NSCLC) who may be eligible for approved targeted therapies at minimal cost. The kit is for in vitro diagnostic use and intended to be used by trained professionals in a laboratory environment.
Principle:
The kit adopts Amplification Refractory Mutation System (ARMS) and real-time PCR technology, which comprises specific primers and fluorescent probes to detect NRAS mutations in human genomic DNA. During the nucleic acid amplification, the targeted mutant DNA is matched with the bases at 3’ end of the primer, amplified selectively and efficiently, then the mutant amplicon is detected by fluorescent probes labeled with FAM. While the wild-type DNA cannot be matched with specific primers, there is no amplification occurs.
Clinical Performance:
The clinical performance of PLC Panel was validated in a concordance study by LC-SCRUM-Japan.1 Results were highly concordant with the reference NGS assay for alterations (fusion, indels, SNVs) across 11 actionable biomarkers.
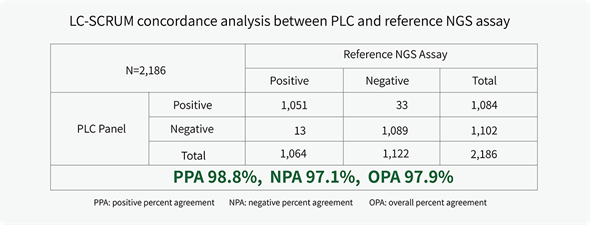
1. Matsumoto, S., et. al. Prospective concordance study of a multi-gene PCR assay and NGS for the detection of targetable gene alterations in lung cancer [abstract] in Journal of Thoracic Oncology. 2021 March; 16:3(S690): WCLC; 2020. Abstract P89.06.
Detected mutations:
EGFR (SNV/INDELS), HER2 (INDELS), KRAS (SNV), BRAF (SNV), ALK (FUSIONS), RET (FUSIONS), ROS1 (FUSIONS), NTRK1 (FUSIONS), NTRK2 (FUSIONS), NTRK3 (FUSIONS), MET (FUSIONS)
Sample requirement:
FFPE or fresh/frozen tissue minimum 67.5 ng DNA; minimum 120 ng RNA
Tests/kit: 8 (preloaded)
Instrument: QuantStudio™ 5, LightCycler® 480 II, cobas z 480
Cart
Payment gate



